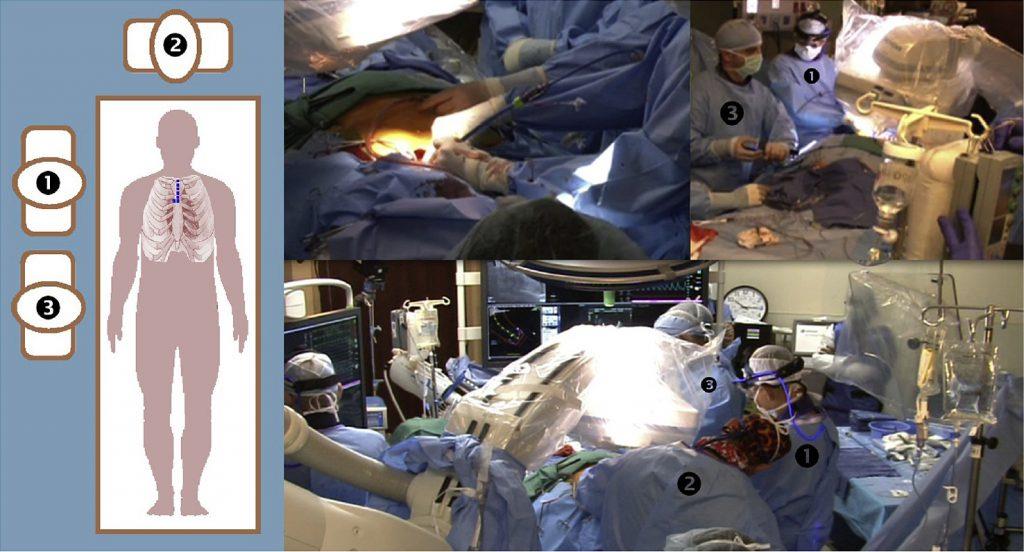
TAVR: TransAortic or TransFemoral?

Editor’s Note:
The last few places I have visited were programs that were scaling up their TAVR programs. There are some pretty exciting and innovative technologies on the rise in the realm of Hybrid technology. Many of us standby for these procedures, I personally have only had to place one patient on emergent CPB- primarily for cardiovascular support to buy time to cardiovert out of a lethal arrhythmia, and then finish the original TAVR procedure.
A few years ago, at the front end of this technology, our TAVR patient population were seriously ill, and this was literally the last ditch effort for survival. As the technology improved, and skill sets developed, there has been a gradual easing of the stringent criteria that patients were required to meet, in order to be offered this surgical option.
Below is an excellent article that discusses patient selection and outcomes for both femoral and aortic TAVR approaches.
Click image above to view source article
Transcatheter aortic valve replacement: Indications
INTRODUCTION — Aortic valve replacement is the mainstay of treatment of symptomatic aortic stenosis (AS). In properly selected patients, this surgical procedure offers substantial improvements in symptoms and life expectancy.
However, aortic valve surgery entails substantial risks for some patients with severe comorbidities, and for some considered at “extreme” risk, surgery is not appropriate. In others, technical limitations, eg, porcelain aorta (extensively calcified ascending aorta and/or aortic arch), may mean that surgery is not feasible. Percutaneous aortic balloon valvotomy was developed as a less invasive means to treat AS but has important limitations. Subsequently developed catheter-based techniques for aortic valve implantation may provide an alternative method for treating AS in patients with unacceptably high estimated surgical risks. A multidisciplinary team approach is recommended in approaching patients with symptomatic AS.
This topic will review indications for transcatheter aortic valve implantation, which has been termed “transcatheter aortic valve replacement” [1]. Indications for aortic valve replacement, surgical aortic valve replacement, estimating the risk of aortic valve surgery, medical therapy of symptomatic AS, and percutaneous aortic valvuloplasty are discussed separately. (See “Indications for valve replacement in aortic stenosis in adults” and “Choice of prosthetic heart valve for surgical replacement” and “Estimating the mortality risk of valvular surgery” and “Medical management of symptomatic aortic stenosis” and “Percutaneous balloon aortic valvotomy”.)
RATIONALE — Transcatheter aortic valve replacement (TAVR) has been developed for the treatment of patients with severe symptomatic aortic stenosis who have an unacceptably high estimated surgical risk, or in whom TAVR is preferred due to technical issues with surgery, eg, a porcelain aorta or prior significant mediastinal radiation, prior pericardiectomy with dense adhesions or prior sternal infection with complex reconstruction, or a patent left internal mammary graft lying beneath the sternum (as identified by computed tomography angiography).
TAVR is now also widely used for the treatment of failed surgical bioprosthetic valves in the aortic position (so-called “valve-in-valve” procedure), and indications include bioprosthetic valve stenosis, regurgitation, or a combination of the two [2].
INDICATIONS
Approach to identifying candidates for TAVR — Indications for transcatheter aortic valve replacement (TAVR) are evolving. Patients may be referred for TAVR for treatment of native aortic valve stenosis, for valve-in-valve treatment of failure of a bioprosthetic aortic valve, or for native aortic valve regurgitation (with the last indication available in Europe but still investigational in North America).
- Among patients with symptomatic native aortic valve stenosis, a choice is made between surgical AVR (SAVR) or TAVR (or no intervention) based upon estimated surgical risk and comorbidities. (See ‘For native aortic valve stenosis’below.)
- A valve-in-valve TAVR procedure is suggested for symptomatic patients with failure (regurgitation, stenosis, or both) of a surgically implanted bioprosthetic valve with high or greater risk for open surgical valve replacement. (See ‘For bioprosthetic aortic valve failure’below.)
- In Europe, a transapical TAVR system (JenaValve) is an option to treat severe native aortic valve regurgitation in patients with high or greater risk for open surgical valve replacement or repair. (See ‘For native aortic valve regurgitation’below.)
For native aortic valve stenosis — Patients with severe native calcific aortic stenosis (AS) are evaluated for presence of an indication for aortic valve replacement. Among patients with severe AS with an indication for valve replacement, a choice is made between SAVR or TAVR (or no intervention) based upon considerations including the estimated surgical risk and comorbidities. (See “Indications for valve replacement in aortic stenosis in adults”.)
Indications for valve replacement for aortic stenosis — The choice of SAVR versus TAVR in patients with severe AS is made after it is determined that the patient meets an indication for valve intervention for severe AS [3]. AVR is the mainstay of treatment of symptomatic AS, as it improves symptoms and prolongs survival. SAVR or TAVR is not indicated in patients with comorbidities that would preclude an expected benefit from correction of AS. (See “Indications for valve replacement in aortic stenosis in adults”, section on ‘Indications for valve replacement’.)
The following are indications for AVR for severe AS that apply to either SAVR or TAVR (table 1 and algorithm 1) [3]:
- AVR is recommended for patients with severe high-gradient AS who have symptoms by history or on exercise testing (stage D1).
- AVR is suggested in symptomatic patients with low-flow/low-gradient severe AS with reduced left ventricular ejection fraction (LVEF; stage D2) with a low-dose dobutaminestress study that shows an aortic velocity ≥4.0 m/s (or mean pressure gradient ≥40 mmHg) with a valve area ≤1.0 cm2 at any dobutamine dose.
- AVR is suggested in symptomatic patients who have low-flow/low-gradient severe AS (stage D3) who are normotensive and have an LVEF ≥50 percent if clinical, hemodynamic, and anatomic data support valve obstruction as the most likely cause of symptoms.
The following are indications for AVR for severe AS that apply only to SAVR (table 1 and algorithm 1):
- AVR is recommended for asymptomatic patients with severe AS (stage C2) and LVEF <50 percent.
- AVR is suggested (weak recommendation) in asymptomatic patients (stage C1) with severe AS and decreased exercise tolerance or an exercise fall in blood pressure.
Choice of therapy — For patients who have severe AS and an indication for valve replacement, treatment decisions are made based upon individualized consideration of the estimated risk and benefit of the procedures, as well as existing comorbidities, including coronary artery disease. For patients being considered for TAVR or high-risk surgical AVR, a multidisciplinary heart valve team (including cardiologists, interventionalists, cardiovascular surgeons, anesthesiologists, and nurses) should collaborate to optimize care [3].
- For symptomatic patients with severe AS who have an indication for AVR and have low surgical risk (ie, STS-PROM <4), we recommend SAVR. (See “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘In low-risk symptomatic patients’.)
- For symptomatic patients with severe AS and an intermediate to high surgical risk (ie, STS-PROM ≥4), clinical studies suggest that either TAVR or SAVR is an option. The decision should be made by a Heart Valve Team with consideration of patient-specific factors (including the feasibility of transfemoral TAVR) and patient values and preferences.
The choice between TAVR and SAVR should also consider whether a bioprosthetic or mechanical valve is most appropriate in each patient. Concurrent conditions, including aortic dilation, coronary disease, and other valve lesions, may also affect this decision. (See “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘In high-risk symptomatic patients’ and “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘In intermediate-risk symptomatic patients’.)
- For symptomatic patients with severe AS, a prohibitive surgical risk, and a predicted post-TAVR survival >12 months, we recommend TAVR rather than medical therapy. Asymptomatic patients with severe AS and prohibitive surgical risk should have frequent clinical evaluation to monitor for symptom onset. (See “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘TAVR versus medical therapy in inoperable patients’.)
- Asymptomatic patients with severe AS who have indications for AVR have heterogenous surgical risk; we recommend SAVR for most of these patients. Choice of treatment for patients at high surgical risk is individualized.
The above approach is similar to that in the 2014 American Heart Association/American College of Cardiology valve guideline (table 2) [3]. However, the recommendation for TAVR with a self-expanding valve in patients with high surgical risk is based upon the United States CoreValve High Risk study, which was published after the guidelines [4]. Recommendations for TAVR in intermediate-risk patients are also based on studies published after those guidelines. (See“Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘In high-risk symptomatic patients’.)
Operative risk assessment (including identification of high and prohibitive risk) includes consideration of estimated operative mortality, frailty, major organ system compromised, and comorbidities (table 3). Accurate estimation of the risk of SAVR performed by an experienced cardiothoracic surgeon and multidisciplinary valve team is vital to appropriate evaluation of potential candidates. Risk assessment for valvular surgery is discussed further separately. (See “Estimating the mortality risk of valvular surgery”.)
Although women experience more major bleeding and vascular complications with TAVR, female sex is an independent predictor of lower mortality after TAVR. Thus, sex-specific mortality risk following TAVR is opposite of that following SAVR, for which women have higher mortality risk than men. While the available data do not establish TAVR as superior to SAVR for women with high-risk symptomatic aortic stenosis, we suggest including female sex as a factor when weighing the potential risks and benefits of TAVR versus SAVR. (See “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘Sex-specific differences in outcomes’.)
Evidence — Evidence to support the above recommendations on choice of surgical or transcatheter intervention for AS is discussed separately.
Recommendations for SAVR for severe AS are based upon comparisons of the natural history of patients with AS with outcomes after SAVR. Although a randomized trial found that outcomes at one year with TAVR and SAVR were similar, we continue to recommend SAVR in patients with low or intermediate surgical risk at this time because the long-term durability (beyond five years) of transcatheter heart valves is not yet known. (See “Indications for valve replacement in aortic stenosis in adults”, section on ‘Evidence’ and “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘In low-risk symptomatic patients’.)
The recommendation for TAVR for patients with AS with prohibitive surgical risk is based upon data from a randomized trial and an observational study with historical control. (See “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘TAVR versus medical therapy in inoperable patients’ and “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘Self-expanding TAVR compared to historical control’.)
The recommendation for SAVR or TAVR in patients with high surgical risk is based upon controlled trials. (See“Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘In high-risk symptomatic patients’.)
For bioprosthetic aortic valve failure — A valve-in-valve procedure is an option for patients with failure (stenosis, regurgitation, or both) of a surgically implanted bioprosthetic aortic valve who are judged by a heart team, including a cardiac surgeon, to be at high or greater risk for open surgical therapy (ie, Society of Thoracic Surgeons operative risk score ≥8 percent or at a ≥15 percent risk of mortality at 30 days) [5]. (See “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘For prosthetic valve dysfunction treated with valve-in-valve’.)
For native aortic valve regurgitation — SAVR remains the treatment of choice for patients with severe native valve aortic regurgitation. When surgery is absolutely contraindicated, TAVR is a potential option. In Europe, a transapical TAVR system (JenaValve) is approved to treat severe native aortic valve regurgitation in patients with high or greater risk for open surgical therapy. Off-label CoreValve use in this setting has been tried but is limited by risk of insufficient anchoring and risk of paravalvular aortic regurgitation. (See “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘For native aortic valve regurgitation’.)
EXCLUSIONS — Patients with a number of conditions are generally excluded from transcatheter aortic valve replacement (TAVR), as recommended in the 2012 American College of Cardiology Foundation/American Association for ThoracicSurgery/Society for Cardiovascular Angiography and Interventions/Society of Thoracic Surgeons expert consensus document on TAVR and the 2012 European Society of Cardiology valve guidelines (table 4) [6,7].
The following exclusion criteria for TAVR are related to the aortic valve:
- Bicuspid or unicuspid or noncalcified aortic valve. While a congenitally bicuspid aortic valve is generally considered an exclusion criterion for TAVR, TAVR has been successfully performed in some patients with this disorder, as discussed separately. Once severe calcific stenosis is present, reliable identification of the number of valve leaflets is problematic, so it is likely that TAVR has been performed in many patients with a congenital bicuspid valve. (See“Management of adults with bicuspid aortic valve disease”, section on ‘Transcatheter aortic valve replacement’.)
- Native aortic annulus size as measured by computed tomography is <18 mm (for a native valve), <17mm (for a surgical valve), or>the largest annulus size for which a TAVR device is available (eg, 29 mm). This criterion is subject to change as the range of available device sizes changes.
Note that valve size numbers do not correspond to actual annular measurements. Deciding which size valve to select for a given manufacturer is complex and is made using computed tomography annulus imaging area and perimeter measurements (with balloon expandable valves defined by area but self-expanding valve defined by perimeter).
- Severe native aortic regurgitation (>3+) is generally an exclusion criterion when TAVR is performed to treat native aortic valve disease; however, the JenaValve has CE Mark approval for use in Europe to treat native aortic valve stenosis and native aortic valve regurgitation.
The presence of severe aortic regurgitation is not an exclusion criterion when TAVR is performed as a valve-in-valve procedure to treat failed bioprosthetic valve.
- As noted above, TAVR is notrecommended for patients with comorbidities that would preclude an expected benefit from correction of AS.
Other relative exclusion criteria that may be used include the following:
- Evidence (such as creatine kinase [CK] plus CK-MB elevation and/or troponin elevation) of an acute myocardial infarction within one month before the intended treatment.
- Hemodynamic or respiratory instability requiring inotropic support, mechanical ventilation, or mechanical heart assistance within 30 days of screening evaluation.
- Need for emergency surgery
- Hypertrophic cardiomyopathy with or without obstruction
- Left ventricular ejection fraction <20 percent
- Severe pulmonary hypertension and right ventricular dysfunction
- A known contraindication or hypersensitivity to all anticoagulation regimens or inability to be anticoagulated for the study procedure.
- Renal insufficiency (eg, creatinine >3.0 mg/dL) and/or end-stage renal disease requiring chronic dialysis
- Echocardiographic evidence of intracardiac mass, thrombus, or vegetation
- Magnetic resonance imaging-confirmed stroke or transient ischemic attack within six months (180 days) of the procedure.
- Severe incapacitating dementia
- Estimated life expectancy <12 months due to noncardiac comorbid conditions
- Severe mitral regurgitation
- Significant aortic disease, including the following abnormalities may preclude a transfemoral approach:
- Thoracic or abdominal aortic aneurysm (luminal diameter ≥5 cm), marked tortuosity (hyperacute bend)
- Aortic arch atheroma (especially if >5 mm thick, protruding, or ulcerated)
- Narrowing (especially with calcification and surface irregularities) of the abdominal or thoracic aorta
- Marked tortuosity (hyperacute bend) of the aorta or severe “unfolding” of the thoracic aorta
SELECTION OF TAVR VALVE TYPE
Patient-specific considerations — For the majority of patients undergoing transcatheter aortic valve replacement (TAVR), either a Sapien, CoreValve, or one of the second generation devices are suitable. For patients treated at a center having sufficient experience with and access to many types of valves, there are certain patient-specific issues that might influence the choice of valve system type:
- Most valves types, but not all, cover the full range of annulus size.
- In a patient deemed to be at high risk of annulus rupture (eg, a patient with a small highly calcified annulus), a self-expanding rather than a balloon-expandable valve may be chosen to reduce the risk of annular rupture (as one of several potential strategies to attempt to reduce the risk of rupture). Annular rupture has been observed almost exclusively after use of a balloon-expandable valve and very rarely after use of a self-expandable valve [8]. (See“Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘Annular rupture’.)
- If there are concerns about coronary obstruction, then a valve system with recapturable technology may be favored.
- When performing a valve-in-valve procedure to treat a small surgical bioprosthetic valve, a supra-annular TAVR valve might offer greater effective orifice area.
Center-specific considerations — Operators have generally selected the type of TAVR valve to implant based upon local practice, operator training, medical center experience, and availability (based upon the regulatory approval status) rather than specific patient-related factors. Individual center procedure volume is an important factor in establishing and maintaining optimum patient outcomes. As a consequence, the majority of centers have implanted only one type of TAVR valve. While that landscape is changing, particularly in countries where there are multiple approved devices, maintenance of sufficient experience with each device used continues to be important for optimum patient outcomes. The differences in patient selection and procedural steps among competing device types are greater for various TAVR systems than for most other interventional cardiovascular procedures.
Regulatory status — Regulatory approvals govern the availability of TAVR technologies.
United States Food and Drug Administration approvals include the following:
- The Edwards SAPIEN XT (balloon-expandable) and the Medtronic CoreValve (self-expanding) systems are approved for patients with symptomatic severe native calcific aortic stenosis who are judged by a heart team, including a cardiac surgeon, to be at high or greater risk for open surgical therapy (ie, Society of Thoracic Surgeons operative risk score ≥8 percent or are judged by the heart team to be at a ≥15 percent risk of mortality at 30 days).
- The Edwards SAPIEN XT and Medtronic CoreValve are also approved for patients with failure (stenosis, regurgitation, or combined) of a surgical bioprosthetic aortic valve who are judged by a heart team, including a cardiac surgeon, to be at high or greater risk for open surgical therapy (ie, Society of Thoracic Surgeons operative risk score ≥8 percent or at a ≥15 percent risk of mortality at 30 days).
A number of TAVR systems have CE mark approval:
- The following devices are approved for use in Europe in patients with severe aortic stenosis at high or greater risk for surgical valve replacement: Medtronic CoreValve, Edwards SAPIEN, Direct Flow Medical (fully repositionable), St. Jude Medical Portico (repositionable prior to deployment), Medtronic Engager (transapical), JenaValve (transapical), Boston Scientific Lotus (repositionable prior to deployment), Medtronic Evolut R (self-expanding and repositionable prior to deployment), and Sapien 3 are approved for patients with severe aortic stenosis with high or greater risk for open surgical valve replacement.
- The CoreValve, Evolut R, and the Sapien XT valve are approved for valve-in-valve use in patients with high or greater risk for open surgical valve replacement.
- The JenaValve transapical TAVR system is also approved to treat severe native aortic valve regurgitation in patients who are inoperable or at high risk for open surgical therapy.
SUMMARY AND RECOMMENDATIONS
- Surgical aortic valve replacement (SAVR) and transcatheter aortic valve replacement (TAVR) are the mainstay of treatment of symptomatic aortic stenosis (AS), as they improve symptoms and prolong survival. SAVR or TAVR are not indicated in patients with comorbidities that would preclude an expected benefit from correction of AS. (See‘Indications for valve replacement for aortic stenosis’above and “Indications for valve replacement in aortic stenosis in adults”, section on ‘Indications for valve replacement’.)
- For patients being considered for TAVR or intermediate- or high-risk SAVR, a multidisciplinary heart valve team (including cardiologist, cardiovascular surgeon, and interventionalist) should collaborate to optimize care. (See‘Choice of therapy’above.)
- While both TAVR and SAVR are effective therapies for severe AS, their complication profiles differ. TAVR entails greater risk of paravalvular aortic regurgitation and major vascular complications. SAVR entails greater risk of major bleeding, acute kidney injury, and new onset atrial fibrillation. (See “Transcatheter aortic valve replacement: Outcomes and complications”.)
- For symptomatic patients who have severe AS, the choice of therapy is based upon consideration of the estimated risk and benefit of the procedures, as well as existing comorbidities.
- For symptomatic patients with AS who have an indication for AVR and have low surgical risk (ie, STS-PROM <4), we recommend SAVR rather than TAVR (Grade 1B). (See “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘In low-risk symptomatic patients’.)
- For symptomatic patients with severe AS and an intermediate to high surgical risk (ie, STS-PROM ≥4), clinical studies suggest that either TAVR or SAVR is an option. The choice between SAVR and TAVR should be made by a Heart Valve Team with consideration of patient specific factors (including the feasibility of transfemoral TAVR) and patient values and preferences. (See “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘In high-risk symptomatic patients’and “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘In intermediate-risk symptomatic patients’.)
- For symptomatic patients with severe AS, a prohibitive surgical risk, and a predicted post-TAVR survival >12 months, we recommend TAVR rather than medical therapy (Grade 1B). (See “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘TAVR versus medical therapy in inoperable patients’.)
- Asymptomatic patients with severe AS who have indications for AVR have heterogenous surgical risk; we recommend SAVR for most of these patients. Choice of treatment for patients at high surgical risk is individualized. (See ‘Choice of therapy’above.)
- For symptomatic patients with failed (stenotic, regurgitant, or both) surgical bioprosthetic aortic valve with high or greater surgical risk, we suggest TAVR rather than SAVR (Grade 2C). (See ‘For bioprosthetic aortic valve failure’above and “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘For prosthetic valve dysfunction treated with valve-in-valve’.)
- In Europe, a transapical TAVR system (JenaValve) is an option to treat severe native aortic valve regurgitation in patients with high or greater risk for open surgical valve replacement or repair. (See ‘For native aortic valve regurgitation’above and “Transcatheter aortic valve replacement: Outcomes and complications”, section on ‘For native aortic valve regurgitation’.)
REFERENCES
- Clegg SD, Krantz MJ. Transcatheter aortic valve replacement: what’s in a name? J Am Coll Cardiol 2012; 60:239.
- Dvir D, Webb JG, Bleiziffer S, et al. Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA 2014; 312:162.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63:e57.
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014; 370:1790.
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfTopic/pma/pma.cfm?num=p130021S010 (Accessed on June 25, 2015).
- Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012; 59:1200.
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS), Vahanian A, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012; 33:2451.
- Park HB, Heo R, ó Hartaigh B, et al. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: a direct comparison to fractional flow reserve. JACC Cardiovasc Imaging 2015; 8:1.