Cold Agglutinins

Literature:

Click image to view source article
Cold Agglutinins in Cardiac Surgery: Management of Myocardial Protection and Cardiopulmonary Bypass
CAs are autoantibodies to RBC antigens [3], which can causesystemic thrombosis and hemolysis. The cause of these CAs maybe primary/idiopathic, or more commonly, secondary to an infectiveprocess (eg, mycoplasma, human immunodeficiency virus) or alymphoproliferative disorder [2]. Reported incidence in screenedcardiac surgery patients is approximately 0.8% to 4% [1], andintraoperative management strategies remain controversial.
CAs are largely asymptomatic and can first present with theinitiation of hypothermic CPB, manifesting as high line pressuresand visible agglutination in the cardioplegia circuit [2, 4].No protection is afforded by anticoagulation or hemodilution.In such cases, immediate management should entail raising thecore temperature to normothermia in conjunction with warm retrogrademyocardial washout. Any resulting hemolysis or end-organ damageshould then be treated.
Patients with low titre, low thermal amplitude CAs can safelyundergo CPB with little change in practice. Preoperative plasmapheresisrequiring high-volume blood product transfusion can achievean eightfold to tenfold reduction in CA titers, with the accompanyingrisks of large volume shifts as well as infection and alteredhemostasis. It should therefore be reserved for those patientsrequiring deep hypothermic arrest [1, 3].
The controversy comes with primary management of patients withhigh titre and high thermal amplitude patients during routinehypothermic CPB. This involves maintaining tissues above thedocumented activation threshold of agglutination by warmingthe operating room and all fluids and anaesthetic gases. Strictcore temperature monitoring should be undertaken, together withthe simple addition of myocardial temperature monitoring toprevent activation of cold agglutinins.
Normothermic CPB with varying techniques of myocardial protectionhave been described in such cases [1–4], including intermittentcross-clamping or induced ventricular fibrillation. Alternatively,some authors have used cold crystalloid cardioplegia alone orthe combination of warm crystalloid cardioplegic washout, followedby cold crystalloid cardioplegia, although this risks microvascularthrombosis from collateral coronary circulation [3].
I. BACKGROUND:
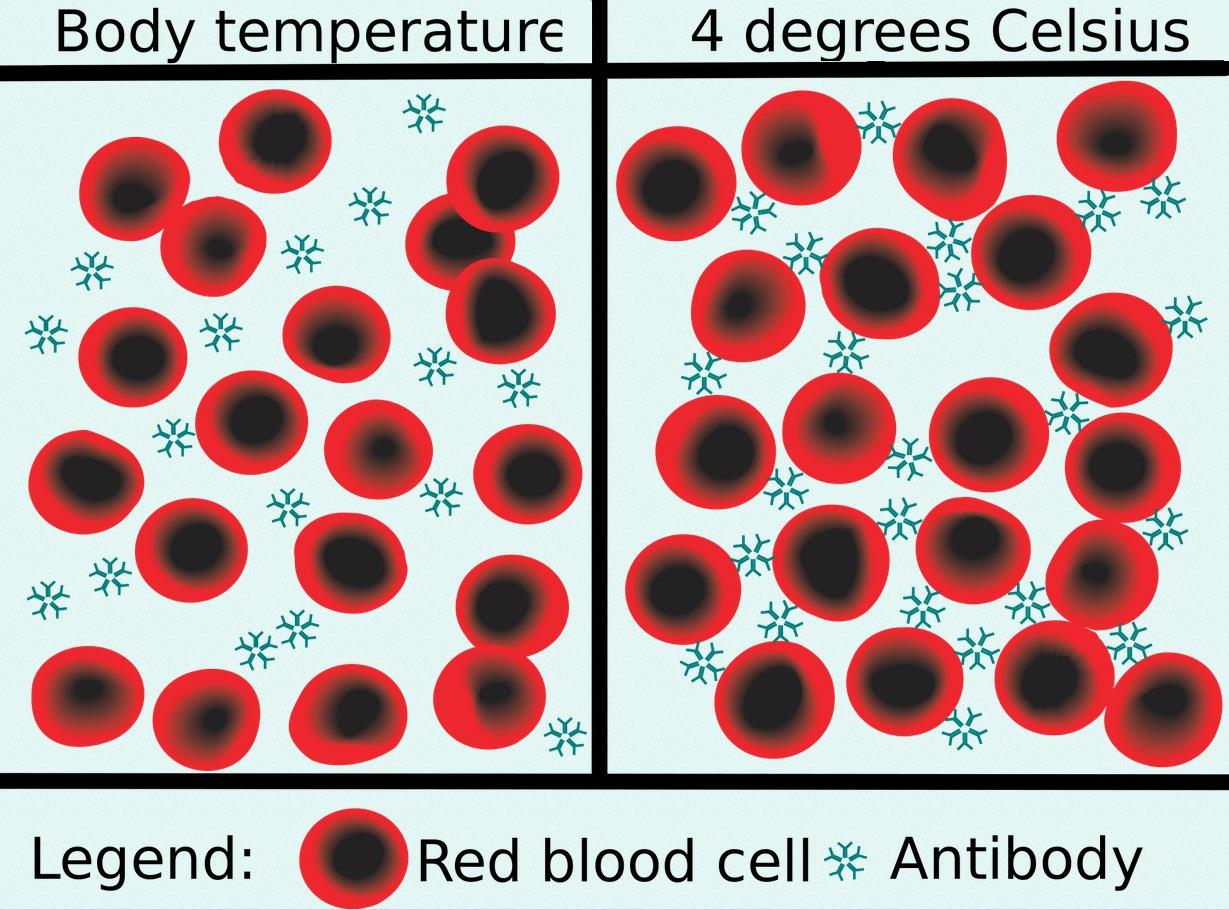
Hypothermic cardiopulmonary bypass with the use of cold blood cardioplegia carries with it the risk of red cell agglutination caused by unsuspected cold agglutinin disease. Patients with this condition possess serum antibodies which become reactive at temperatures usually less than 30°C and are directed against an antigen of the human erythrocyte causing agglutination and/or hemolysis. The clumping phenomenon is do to diminished negative surface charges while hemolysis depends on the extent of complement activation.
This autoimmune phenomenon is fairly rare and may be naturally occurring or acquired in association with viral infections, lymphoproliferative disorders and other autoimmune disorders. The idiopathic form has peak incidence after age 50. Those associated with recent viral infections are often transient thus such patients should be tested for their antibody titer and surgery could be postponed for two to three weeks if possible. The titer is the greatest dilution in saline at which the antibody is reactive.
Most blood bank pretransfusion testing is not designed to identify cold antibodies, particularly those of low thermal amplitude. Cold agglutination disease is frequently asymptomatic and unsuspected. Pretransfusion testing on freshly obtained specimens normally occurs at room temperature. Furthermore, cold antibodies are rarely active at more than 30°C and usually less than 25°C.
Unexpected cold agglutination is thus normally only observed in the cardioplegia administration set when blood is used. There is however at least one report of clumping observed in a cardiotomy reservoir at 30°C. In vitro, agglutination appears reversible on rewarming, but in vivo, it can cause capillary sludging leading to gangrene and ischemia. In the case of cold blood cardioplegia sludging might interfere with cardioplegia distribution resulting in poor myocardial protection and depressed left ventricular function.
II. RECOMMENDATIONS:
As is most often the case, when observed unexpectedly, the surgery team should immediately switch to an alternative method of myocardial protection. The perfusionist should communicate his observations to the surgeon and anesthesiologist so they may decide on an option.
Following is an outline of some different options that have been used successfully by others. Ideally these options could be planned in advance for a patient with a known critical temperature. However, they should be effective if switched to in the acute agglutination setting since deagglutination occurs upon rewarming.
A. Option 1: Continuous Warm Blood Cardioplegia
The recently popular technique of warm continuous retrograde cardioplegia is ideal for use on patients with cold antibodies. The body temperature can be maintained at no less than 35°C as is the cardioplegia temperature. When on CPB filling of the right atrium will help distend the coronary sinus and facilitate placement of the retrograde cannula. Problems with switching to this technique are as usual with retrograde. Namely, the inability to place and keep the retrograde cannula in the coronary sinus and difficulty with visualization at the anastomosis site.
Continuous antegrade is a variation of this technique of myocardial protection both down vein grafts and into the aortic root. The problem with this however, is the fast rate of continuous infusion to keep the aortic valve competent. Thus, this variation alone is best reserved for aortic valve replacement when the ostium could be directly cannulated. When doing so the cardioplegia line can be “Y’d” off at the table to perfuse both ostium simultaneously. Keep in mind that normal resting coronary flow is about 250cc/min and perfusion pressure in the coronary arteries is normally 80-100mmHg.
B. Option 2: Intermittent Cardiac Ischemia with Moderate Cardiac Hypothermia
This technique assumes that the critical temperature is less than 28°C which is most often the case. This should, however, be determined by the lab as quickly as possible. With the systemic body temperature maintained as 28°C blood cardioplegia is given also at 28°C. This necessitates a stricter time limit between doses. Successful limits up to 30 minutes at 28°C and 45 minutes at 22°C myocardial temperatures have been documented if full arrest is maintained. However, 10 to 15 minutes is probably the safest range at 28°c. The heart must be kept still however. Myocardial O2 consumption has been documented to be almost seven times greater at 22°C in a beating or fibrillating heart than in a quiescent heart. Once the critical temperature is known then further protection is afforded by lowering the systemic blood temperature more (i.e. 28°C to 22°C) or by using a slow cardioplegia water pump speed and closely monitoring the outlet temperature.
A variation of this option would be to use intermittent cross clamp periods in order to reperfuse the heart for 3 to 5 minutes at about 15 minute intervals. The heart is made to beat not fibrillate during this time. This was common practice between 1960 to 1975. The problem with this is even a 22°C the heart must often be electrically fibrillated for good surgical exposure. Also, the risks of reperfusion injury and air embolization are greater. Lastly, although good clinical results were obtained, this variation is less effective in theory because of the increased oxygen demands during fibrillation.
C. Option 3: Warm Wash Out Followed by Cold Crystalloid Cardioplegia
This technique is reported as being most effective when the heart is totally isolated from the systemic circulation. Thus bicaval cannulation with tourniquets is recommended. Also an insulation pad under the heart is recommended. To use only crystalloid the 1/4″ line is clamped and taken out of the plegia pump raceway. A bushing can be formed with tape around the 1/8″ line to prevent creep into the raceway. Flow will then be half that indicated on the 1/4″ scale of the speed dial. Thus 50 RPM’s rather than 25 RPM’s will provide approximately 330cc/min flow. The cardioplegia manifold is warmed to 37°C and patient only mildly cooled or allowed to drift (33 to 35°C). This of course may be decreased if and when the critical temperature is known. If blood is already in the system it can be displaced first to eliminate clumped cells in the lines or the system can be completely changed. A sump catheter should also be placed in the right atrium to collect the effluent. If bicaval cannulation is used ahead of time the atrium can be opened and suctioned out. Once these are ready the aorta is cross clamped and 37°C crystalloid cardioplegia (20mEq KCL/L) is used to flush out the coronary circulation. This will take about three minutes and should be continued until the fluid from the right atrium is clear. This should not be returned to the oxygenator. A low vacuum can be sued to gently aspirate effluent from the right atrium. Infusion is then continued with more of the same solution but cooled to <10°C until no electrical activity is seen. Venting of the aorta or left ventricle will reduce non-coronary collateral flow. Cold intermittent doses should be given as would be with cold blood and will help maintain hemodilution and washout of the coronaries. Before cross clamp removed a final 37°C warm flush is given and the septal temperature monitored to insure that it exceeds the critical temperature of the cold antibody.
D. Other recommendations are:
1. Keep transfusion of blood products to a minimum to avoid introducing fresh complement components. This includes platelet infusions.
2. If infusions are required, they should be warmed post-operatively.
3. Another strategy for dealing with cold antibodies, if known before hand, is plasma exchange. Preoperative plasmapheresis alone can be performed to temporarily reduce cold agglutinin titers. It has been shown that as much as 90% of the offending cold agglutinin may be removed and enable the team to employ a more severe hypothermic protocol than would otherwise be possible.
4. Excessive hemodilution should be considered if at, or going to, cold temperatures. This will effectively dilute the concentration of the cold agglutinin antibody titer. The excess fluid may be removed after rewarming to a safe temperature.