AngioVac

Looking for Written protocols
Anybody have any Experience with this Technology?
(Click Image to View Website)
I got a Facebook Question today…
“Do you know of any written protocols for the Angiovac? I have emailed the company and asked for an inservice and protocols and have not heard back from them.
Thanks,
Michelle”
The Company Literature …
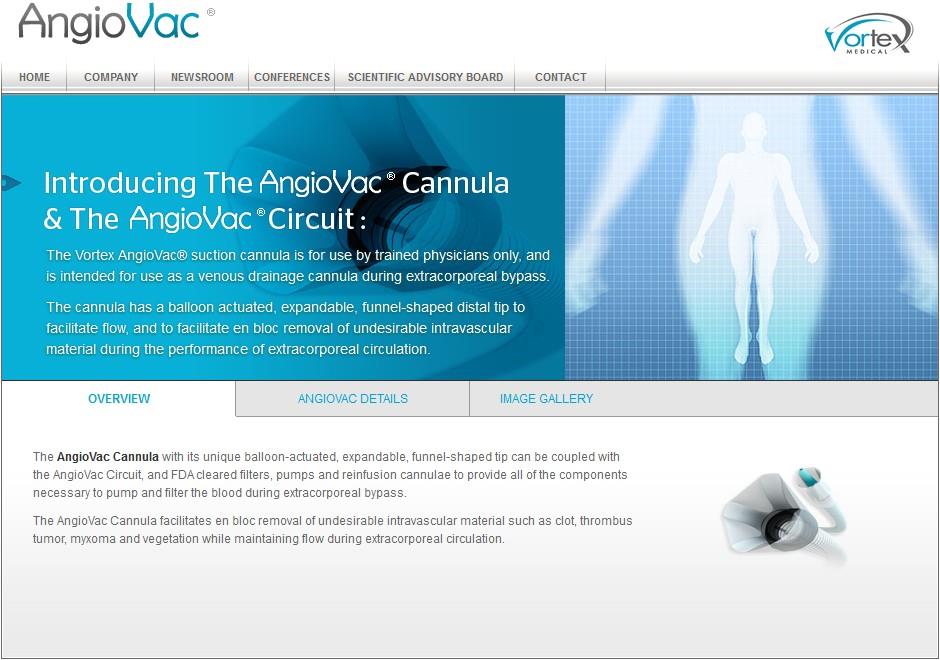
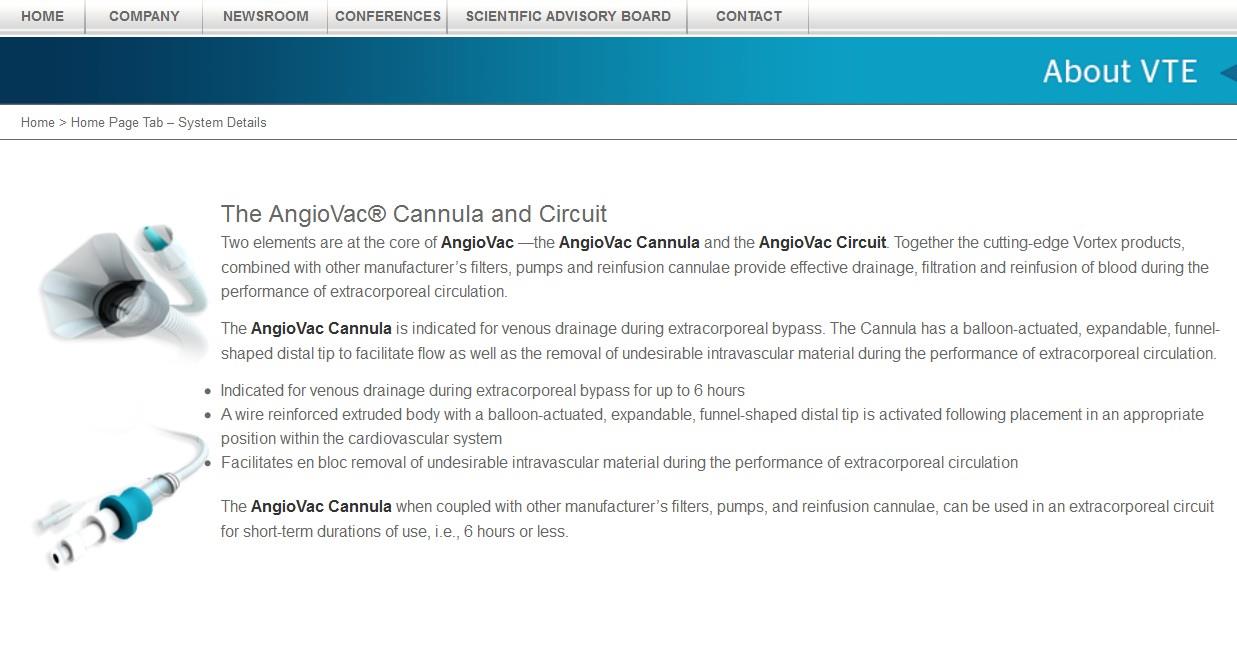
Two elements are at the core of AngioVac —the AngioVac Cannula and the AngioVac Circuit. Together the cutting-edge Vortex products, combined with other manufacturer’s filters, pumps and reinfusion cannulae provide effective drainage, filtration and reinfusion of blood during the performance of extracorporeal circulation.
The AngioVac Cannula is indicated for venous drainage during extracorporeal bypass. The Cannula has a balloon-actuated, expandable, funnel-shaped distal tip to facilitate flow as well as the removal of undesirable intravascular material during the performance of extracorporeal circulation.
- Indicated for venous drainage during extracorporeal bypass for up to 6 hours
- A wire reinforced extruded body with a balloon-actuated, expandable, funnel-shaped distal tip is activated following placement in an appropriate position within the cardiovascular system
- Facilitates en bloc removal of undesirable intravascular material during the performance of extracorporeal circulation
I personally am unfamiliar with the system or the technology. I looke up the company website, and also searched for some comments, so that’s what we have her.
I’ll leave the company a message to see if they can offer a little more help as well.
If any of you have any ideas or suggestions or actual protocols…
Well here is my Facebook Link– and maybe you contact Michelle (or CircuitSurfers) and share what you know?
Any help is totally Appreciated 🙂
Frank